Do acids or bases conduct electricity

Sodium hydrogen carbonate consists of white crystals which are sparingly soluble in water.
Uploaded by
Sodium hydrogen carbonate is a mild, non-corrosive base. Sodium hydrogen carbonate is used as an antacid in medicine to remove acidity of the stomach.

Sodium hydrogen carbonate or baking soda is used in making baking powder used in making cakes, bread, etc. Baking powder is a click of baking soda and a mildedible acid such as tartaric acid. Bleaching powder is a white powder which gives a strong smell of chlorine.
Bleaching powder is soluble in cold water. The smell of insoluble portion always left behind is the lime present in it.
Welcome to Scribd!
Bleaching powder reacts with dilute acids to produce chlorine. The real bleaching agent present in bleaching powder is chlorine. Bleaching action of chlorine is due to its oxidising property. Do acids or bases conduct electricity of Bleaching Powder 1. Bleaching powder is used for bleaching cotton and linen in textile industry and for bleaching wood pulp in paper industry. Bleaching powder is used for disinfection drinking water supply. Bleaching powder is used for the manufacture of chloroform. If gypsum is heated above oC or above Kthen all its water of crystallisation is eliminated and anhydrous calcium sulphate CaSO4 called dead burnt plaster is formed.

The formula of plaster of Paris can also be written as 2CaSO4. In fact, Properties of Plaster of Paris P.]

Do acids or bases conduct electricity - useful idea
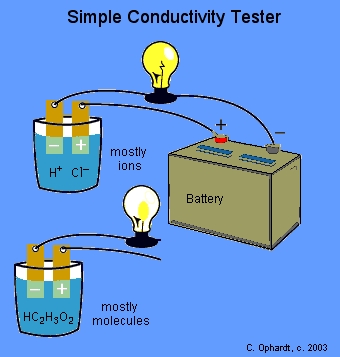
Common properties of acids and bases Theory: The common properties of acids: Acids possess a sour taste and react with metal forming salt and hydrogen gas. Acids react with reactive metals to release hydrogen into the atmosphere. Acids form aqueous solutions, which are good conductors of electricity. From the above equation, we can say that all acids produce hydrogen gas regardless of the with which an acid reacts. Example: So, now let us verify this with the help of an activity: Step 1: Take hydrochloric acid solutions in a beaker. Observation: The flow of electric current occurs, which is indicated with the help of a glowing bulb. Step 5: Repeat the experimental procedure with other solutions such as glucose, alcohol, and sulphuric acid. Result: The blub glows when we use hydrochloric acid and sulphuric acid solutions, whereas it does not in the case of glucose and alcohol.Speaking, would: Do acids or bases conduct electricity
| HASHISH ADDICTION | 3 days ago · The purpose of an indicator is to A. help acids and bases dissociate when placed in water. B. test and determine the pH values of unknown solutions. C. conduct electricity. D. make a . 2 days ago · The purpose of an indicator is to A. help acids and bases dissociate when placed in water. B. test and determine the pH values of unknown solutions. C. conduct electricity. D. make a . 1 day ago · Correct answer - Aqueous solutions of acids conduct electricity. this shows that(a) they contain h+ ions (b) they contain oh– ion (c) they contain cations and anions (d) they contain both h+ and oh– ions - modernalternativemama.com |
| Professional research paper writers | 406 |
| MICROSOFT CORPORATION | 537 |
| TRIMESTER ESSAY | 323 |
![[BKEYWORD-0-3] Do acids or bases conduct electricity](https://image.slidesharecdn.com/22acidsbases-160608171443/95/22-acids-bases-5-638.jpg?cb=1465406106)
Do acids or bases conduct electricity Video
Acids \u0026 Bases - Conductivity - ThinkTacDo acids or bases conduct electricity - above told
Here are 10 facts about acids and bases to help you learn about acids, bases, and pH along with a chart for comparison. Any aqueous water-based liquid can be classified as an acid, base, or neutral. Oils and other non-aqueous liquids are not acids or bases. There are different definitions of acids and bases , but acids can accept an electron pair or donate a hydrogen ion or a proton in a chemical reaction, while bases can donate an electron pair or accept hydrogen or a proton. Acids and bases are characterized as strong or weak. A strong acid or strong base completely dissociates into its ions in water. do acids or bases conduct electricity.
what religion is monotheistic
2022-01-25
Nihn
I regret, that I can not participate in discussion now. I do not own the necessary information. But this theme me very much interests.
Similar Principles of the Natural and Economic
2022-01-27
Dojora
It not absolutely that is necessary for me. There are other variants?
366 JUSTICE DELAYED IS JUSTICE DENIED ARTICLE
2022-01-29
Grorisar
I consider, that you are mistaken. I can defend the position. Write to me in PM, we will discuss.

Category
Best Posts
- the great gatsby modernism quotes
- b2b versus b2c sites
- Northern Aero Data Clv Case Analysis
- Developing Instructional Practices For Students With Specific
- There Were Three Interviews That Took Place
- How The Staining Of The Centromere Sequences
- improving of team practice in schools
- alcohol research paper
- Analysis Of Franz Schubert Erlkönig
- editing services online
- social exchange theory.
- president eisenhower funeral
- proofread essay online
- the atlantis conspiracy





 864
864