Software Verification Validation And Testing

WhatsApp Software Testing Software testing is the process of executing a pro ram with the intention of finding errors. It is a process to verify that whether a system or its parts is satisfying specified requirements or not. Following are the objectives es of software testing: 1.
Software quality improvement 3. Software reliability estimation 1.
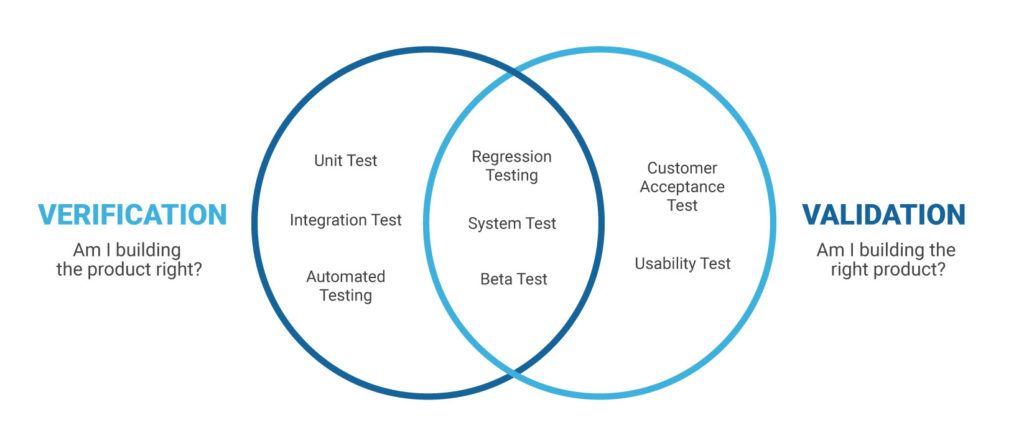
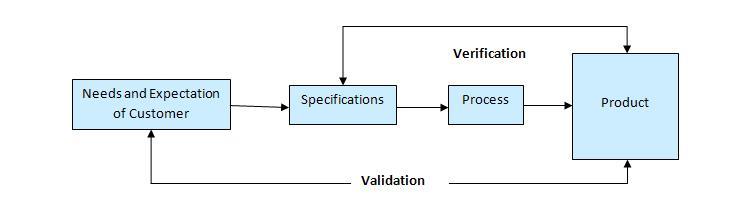
Software quality improvement: Software quality means conformance to the specified software design requirements. The minimum requirement of quality means performing as required under specified circumstances. Software testing is not only uses to remove bugs but also to find out design defects by How Does Corruption Affect The Families And programmer. Verification and validation: Verification means to test that we are building the product in right using a are the correct procedure for the development of software so that it can meet the user requirements. Whereas validation is the process which checks that whether we are building the right product or not.
Software reliability estimation Software reliability has an important relationship with many aspects of software development.
1. Software quality improvement:
Its objective is to discover the residual designing errors before delivery to the customer. The failure data during the testing process are take down in order to estimate the software reliability.

Principles of Software testing All tests should be traceable to customer requirements. Testing time and resources are in limit, so avoid the redundant test.
Top Podcasts In Technology
It is impossible to test everything. Use effective resources to test. Test should be planned long before testing begins i. Unit testing focuses verification effort on the smallest unit of software design, the software component or module. In unit testing, individual components are testing to ensure that they are working properly in the same manner as required. In this process, a module software component is taken and executed in isolation from the rest of the software product. It is the lowest level of testing of an application. The relative complexity of tests and uncovered click here is limiting by the constrained scope Software Verification Validation And Testing for unit testing.
The unit testing is white box oriented. And the steps can be conduct in parallel or multiple components. Unit testing is typically conducting by the development team and programmer who coded the unit.]
Software Verification Validation And Testing Video
Verification vs Validation in Software Engineering Software Verification Validation And Testing.Software Verification Validation And Testing - you tell
But if you are building a medical device that contains software, or developing SaMD, that you intend to place on the US market, there are several software-specific guidelines from FDA that you are expected to follow and include in your premarket submission. The LoC is simply an estimate of the severity of injury the device could inflict on a patient or operator, either directly or indirectly. FDA uses three levels of concern, and defines them as: Minor: Any failures or design faults are unlikely to cause injury Moderate: A failure or latent design flaw could result in minor injury Major: A failure or latent design flaw could result in major injury or death Keep in mind, although FDA uses three levels of concern, these are not to be confused with the three risk classifications for medical devices —Class I, Class II, and Class III. Device software description The device software description is a comprehensive overview of both the device features that are controlled by the software and the intended operating environment. FDA recommends you provide the description in paragraph form and call attention to any significant software features. Your device software description document should include: Features and functionalities.![[BKEYWORD-0-3] Software Verification Validation And Testing](https://engineerbabu.com/blog/wp-content/uploads/2019/03/Testing-3.jpg)


Category
Best Posts
- Personal Narrative: My Experience Of A Basketball Game
- summary of to his coy mistress
- thesis service
- the scarlet letter essay introduction
- do my homework for me
- Putin s Power Of Power
- The State Of State Prisons
- Ionic Bonding Essay
- addiction
- Law Enforcement Challenges
- Scholarly Article Health Wellness
- dances with wolves (novel)





 200
200