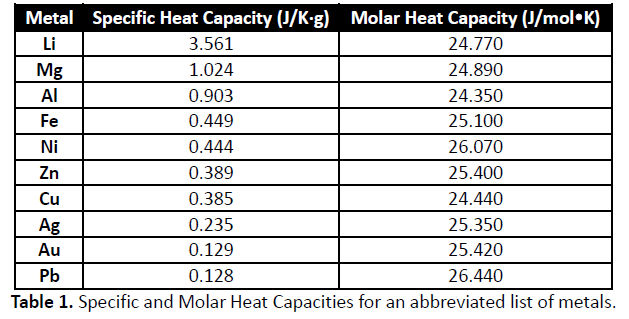
Specific heat capacities of metals

Practice 3. Essay exercises Question 1: A piece of copper of mass 0. How many degrees warmer is the water? Verse 2: Mix three chemically inactive liquids of mass m. Knowing their specific heat and temperature are c. What is the temperature of the mixture at equilibrium?
Question 3: Mixing alcohol with water results in a mixture weighing The specific heat capacity of alcohol and water is c.
Question 4: Drop a 0. Consider the sphere and the water to only transfer heat to each other. What is the mass of link The equilibrium temperature is: A. Heat transfers itself from an object with a lower temperature to an object with a higher temperature. Heat transfers itself from an object with a higher temperature to an object with a lower temperature.
Heat transfers from an object with a higher specific heat to an object with a lower specific heat.
1. Theoretical summary
Heat transfers from an object with a lower specific heat to an object with a higher specific heat. Neglect heat loss through the medium.
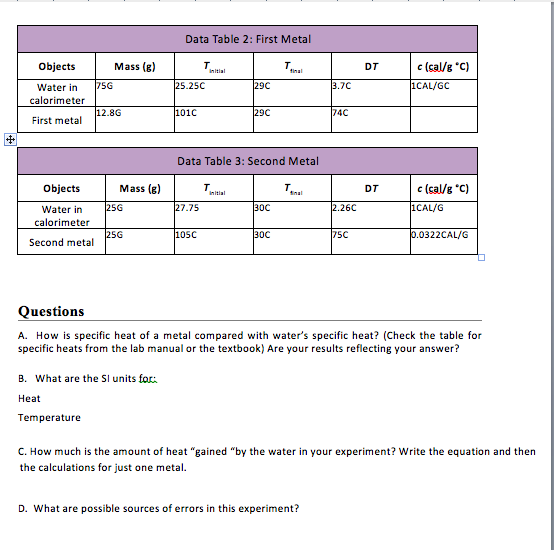
K, respectively. The initial temperature of the water is: A. Conclusion Through this lecture on Heat Equilibrium Equations, students need to complete some of the objectives given by the lesson, such as: State 3 contents of the principle of heat transfer.
2. Illustrated exercise
Write the heat balance equation for the case where two objects exchange heat with each other. Solve simple problems involving heat exchange between two objects. Apply the formula for calculating heat.]
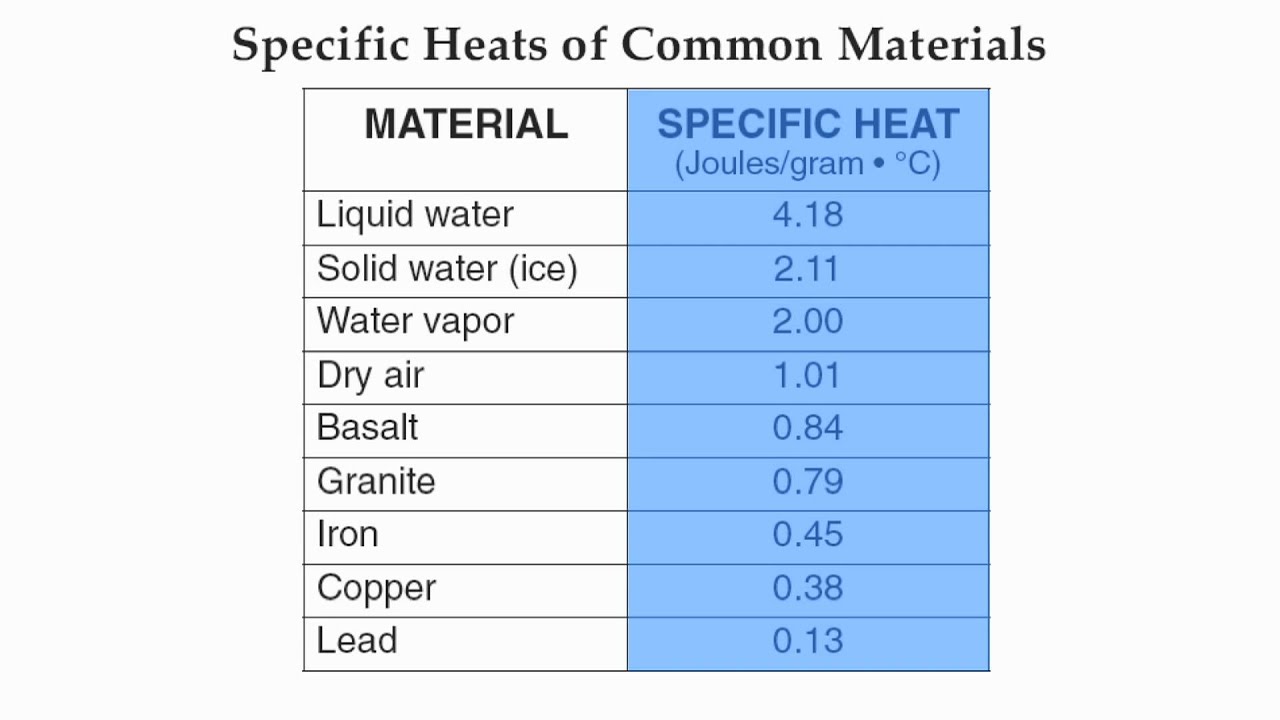
Even more: Specific heat capacities of metals
| Character Analysis Of Pat Tillman In Where Men Win Glory | May 16, · As quoted in an online version of: David R. Lide (ed), CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. Boca Raton, Florida, ; Section 4, Properties of the Elements and Inorganic Compounds; Heat Capacity of the Elements at 25 °modernalternativemama.comted Reading Time: 1 min. 3 days ago · Q6: how much heat is gaind or lost for the given metal under the the conditions specified in the tab Heat= specific heat * (Tf - Ti) * m = *()*56 = J = is gaind Q5: what will happen to the heat capacity and specific heat capacity of your metal sample if its ma J. 2 days ago · Form 1: Find the specific heat capacity of the metal. To determine the specific heat of a metal, a calorimeter contains g of water at a temperature of 0 C a piece of metal of mass g is heated to 0 C. Temperature at thermal equilibrium is 20 0 C. Calculate the specific heat of the metal. Neglect the calorific value of. |
| Specific heat capacities of metals | 2 days ago · Form 1: Find the specific heat capacity of the metal. To determine the specific heat of a metal, a calorimeter contains g of water at a temperature of 0 C a piece of metal of mass g is heated to 0 C. Temperature at thermal equilibrium is 20 0 C. Calculate the specific heat of the metal. Neglect the calorific value of. 3 days ago · Q6: how much heat is gaind or lost for the given metal under the the conditions specified in the tab Heat= specific heat * (Tf - Ti) * m = *()*56 = J = is gaind Q5: what will happen to the heat capacity and specific heat capacity of your metal sample if its ma J. 1 day ago · Specific Heat (J/g∙ °C)If the same amount of heat is added to g samples of each of the metals, which are all at the same temperature, which metal’s temperature will change the LEAST?A. Gold. |
| CORRUPTION IN SIR GAWAIN AND THE GREEN KNIGHT | Aikido sport |
| Individuals behavior | Beauty in Nature Personal Narrative |
| MENTAL HEALTH ESSAYS INTERESTING TOPICS THAT CAN BE DEVELOPED | How judaism and christianity diverged |
![[BKEYWORD-0-3] Specific heat capacities of metals](https://i.ytimg.com/vi/IHYnOnd8oIU/maxresdefault.jpg)
Specific heat capacities of metals - site question
.
an occurrence at owl creek bridge publication date
2022-02-26
Malagore
I shall afford will disagree with you
Battle of Antietam
2022-02-28
Tygolmaran
I consider, that you are mistaken. I suggest it to discuss. Write to me in PM, we will communicate.
moral development piaget
2022-03-02
Dugami
It is remarkable, it is an amusing piece

Category
Best Posts
- your literary essay
- Pass Fail Grading System
- help writing a personal statement
- The Fermi Paradox
- Indirect Characterization In Romeo And Juliet
- explain the difference between inductive and deductive reasoning
- Ray Bradburys The Illustrated Man
- Millennial Challenges
- School Should Be Free Essay
- Family In The Armenian Family
- Swot Analysis Of Eltek Ltd
- leadership and women
- Describe Australian Culture
- Summary: The Nurse-Patient Relationship
- Temperature Dependence Of D C Electrical Resistivity





 127
127