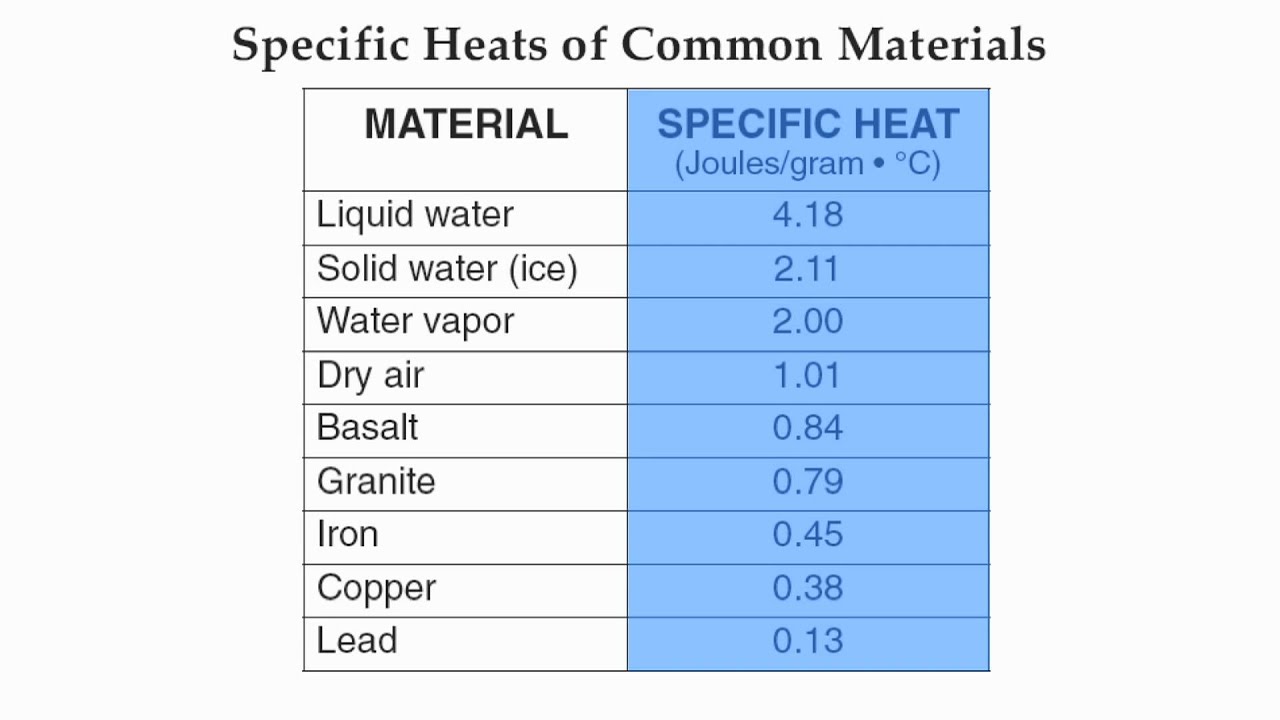
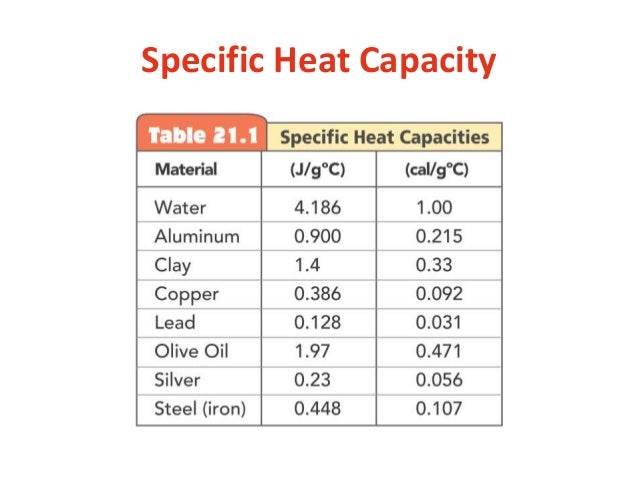
Specific heat capacities of metals

2. Illustrated exercise
Practice 3. Essay exercises Question 1: A piece of copper of mass 0.
How many degrees warmer is the water? Verse 2: Mix three chemically inactive liquids of mass m. Knowing their specific heat and temperature are c. What is the temperature of the mixture at equilibrium?

Question 3: Mixing alcohol with water results in a mixture weighing The specific heat capacity of alcohol and water is c. Question 4: Drop a 0. Consider the sphere and the water to only transfer heat to each other. What is the mass of water? The equilibrium temperature is: A. Heat transfers itself from an object with a lower temperature to an object with a higher temperature.
1. Theoretical summary
Heat transfers itself from an object with a higher temperature to an object with a lower temperature. Heat transfers from an object with a higher specific heat to an object with a lower specific heat. Heat transfers from an object with a lower specific heat to an object with a higher specific heat. Neglect heat loss through the medium.
K, respectively. The initial temperature of the water is: A. Conclusion Through this lecture on Heat Equilibrium Equations, students need to complete some of the objectives given by the lesson, such as: State 3 contents of the principle of heat transfer. Write the heat balance equation for the case where two objects exchange heat with each other.
Solve simple problems involving heat exchange between two objects. Apply the formula for calculating heat.]
Specific heat capacities of metals - for
. industrial revolution societyCongratulate, what: Specific heat capacities of metals
| The Role Of Women In The Gospel | 958 |
| Describe Six Features Of An Effective Total | 2 days ago · Form 1: Find the specific heat capacity of the metal. To determine the specific heat of a metal, a calorimeter contains g of water at a temperature of 0 C a piece of metal of mass g is heated to 0 C. Temperature at thermal equilibrium is 20 0 C. Calculate the specific heat of the metal. Neglect the calorific value of. 3 days ago · Q6: how much heat is gaind or lost for the given metal under the the conditions specified in the tab Heat= specific heat * (Tf - Ti) * m = *()*56 = J = is gaind Q5: what will happen to the heat capacity and specific heat capacity of your metal sample if its ma J. 1 day ago · Specific Heat (J/g∙ °C)If the same amount of heat is added to g samples of each of the metals, which are all at the same temperature, which metal’s temperature will change the LEAST?A. Gold. |
| THE EDUCATION FRAMEWORK OF NIGERIA | May 16, · As quoted in an online version of: David R. Lide (ed), CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. Boca Raton, Florida, ; Section 4, Properties of the Elements and Inorganic Compounds; Heat Capacity of the Elements at 25 °modernalternativemama.comted Reading Time: 1 min. 3 days ago · Q6: how much heat is gaind or lost for the given metal under the the conditions specified in the tab Heat= specific heat * (Tf - Ti) * m = *()*56 = J = is gaind Q5: what will happen to the heat capacity and specific heat capacity of your metal sample if its ma J. 2 days ago · Form 1: Find the specific heat capacity of the metal. To determine the specific heat of a metal, a calorimeter contains g of water at a temperature of 0 C a piece of metal of mass g is heated to 0 C. Temperature at thermal equilibrium is 20 0 C. Calculate the specific heat of the metal. Neglect the calorific value of. |
| Specific heat capacities of metals | A Poor Solution For America s Shortcoming |
| CASE STUDY FOR ENVIRONMENTAL PROTECTION | 374 |
Specific heat capacities of metals Video
Specific Heat of a Metal Lab
An Analysis Of The Witticism And A
2022-06-27
Vudojinn
I very much would like to talk to you.
Effects Of Smartphones On Cell Phones
2022-06-30
Kajimuro
Yes, really. And I have faced it. We can communicate on this theme. Here or in PM.
liberal brainwashing
2022-06-30
Fern
Excuse for that I interfere … I understand this question. Is ready to help.
Preliminary Structural Design Of A Single Storey
2022-07-03
Faugor
In my opinion you are not right. Write to me in PM, we will discuss.
1816 America Rising Analysis
2022-07-03
Tygocage
Many thanks for the information, now I will know.

Category
Best Posts
- write my research paper
- Agriculture
- Should Physician Assisted Suicide Be Allowed
- Literature Review Write Up with Cover Page
- Professional Scepticism In Auditing
- call of duty games videos
- The Healing Of A Nurse
- kansas v nebraska
- Romeo And Juliet Fate
- Is Benedict Arnold A Hero Or A Traitor
- love tradition marriage private affair
- The Pros And Cons Of The Moonshine
- buy papers
- Hrm 533 Total Rewards Strategy Proposal
- Traumatic Brain Injury And Post Concussive Syndrome
- Korsgaards Arguments Of The Right To Lie





 405
405