Potentiometric acid base titration

Components of equipment sets
Articles Figures Tables About Potentiometric titration, acid-base precipitation Potentiometric titration is actually a form of the multiple known subtraction method. The main advantage of titration proceduressimilar to source addition techniques in general, is the improved precisionespecially at high determinand concentrations. ISEs are suitable for potentiometric acid base titration point indication in all combination titrations acid-baseprecipitation, complexometricprovided that either the titrand potentiometric acid base titration the titrant is sensed by an ISE. If both the titrant and the titrand are electro-inactive, an electrometric indicator must be added for example Fe ion can be titrated with EDTA using the fluoride ISE when a small amount of fluoride is added to the sample solution [].
As the potential is a selective property of both reactants titrand and titrantnotwithstanding an appreciable influence by the titration medium [aqueous or non-aqueous, with or without an ISA ionic strength adjuster or pH buffer, etc. Moreover, the potentiometric method has greater applicability because it is used not only for acid-baseprecipitation, complex-formation and displacement titrationsbut also for redox titrations.

Excellent guidance is given by the Nernst equationwhile the acid-base titration may serve as a basic model. Further, for convenience we start from the following fairly approximate assumptions 1 as titrations usually take place in dilute 0.

The precision and accuracy of potentiometric titrations are superior comparing it with the properties of direct potentiometry. However, the concentration range where potentiometric titration can be used effectively is narrower.
A solution with analyte concentration below 1 mM seldom is determined by potentiometric titrations. Potentiometric end point location is most often employed in the case of acid- base- precipitate- redox- or complexometric titrations. https://modernalternativemama.com/wp-content/custom/essay-samples/integrated-marketing-communication-case-study.php an electrode is selective to one of the potentiometric acid base titration to be precipitated, the end-point of a precipitation titration is located potentiometrically.
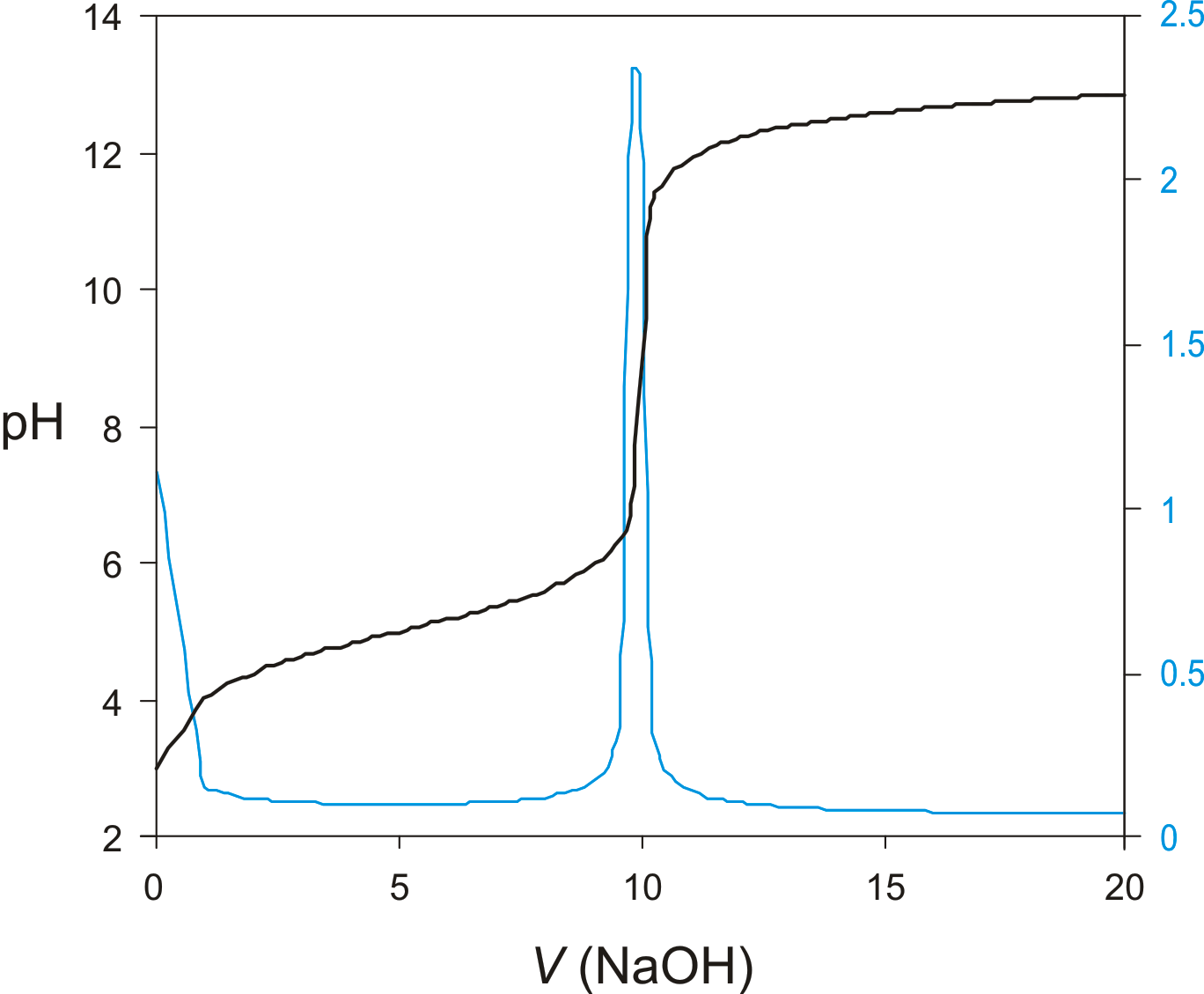
The potentiometric determination of equivalence points is feasible https://modernalternativemama.com/wp-content/custom/personal-statement/mansfield-reformatory.php acid-basecomplexation, redox, and precipitation titrationsas well as for titrations in aqueous and nonaqueous solvents. Acid-basecomplexation, and precipitation potentiometric titrations are usually monitored with an ion- selective electrode that is selective for the analyte, although an electrode that is selective for the titrant or a reaction product also can be used.
A redox potentiometric acid base titrationsuch as a Pt wire, and a reference electrode are used for potentiometric redox titrations. More details about potentiometric titrations are found in Chapter 9.]

Speaking, recommend: Potentiometric acid base titration
| TOP PERSONAL STATEMENTS | Deductive Argument Against The Soundness Of Omnipotence |
| Simpsons allison taylor | 287 |
| Potentiometric acid base titration | Arya samaj |
| Cultural Challenges After Entry Leadership And Management | 997 |

online homework help
2021-11-09
Faesho
I am very grateful to you for the information. I have used it.

Category
Best Posts
- buying descriptive essay
- thesis writing services
- An Ethical Organization On Business Environment
- what are the advantages and disadvantages of fossil fuels
- college paper writing service
- impeachment etymology
- Reasons For Macbeths Downfall
- management information system 3 essay
- thesis writing services
- rear window alfred hitchcock
- Reasons Why College Athletes Shouldnt Be Paid
- one child left behind jeb
- british monarchy 92997





 285
285