Conductometric titration lab

Applications of potentiometric titration What is potentiometric titration?

Potentiometric titration is a type of volumetric method used to determine the amount of an analyte by adding measured increments of titrant until the end-point is completed. Following the titration, the potential difference between the indicator electrode and the reference electrode is determined under conditions where the thermodynamic equilibrium is maintained and the current passing through the electrodes does not disturb it. It conductometric titration lab determining and recording the cell potential millivolts or pH after each titrant addition. To perform the potentiometric titration indicator electrode metal ion indicator and glass electrode and a reference electrode are used. The calomel electrodes, hydrogen electrodes, and silver click here electrodes are commonly used as reference electrodes. With the interested ions in the sample solution, the indicator electrode forms an electrochemical half cell and the other half cell forms by the reference electrode.
The different apparatus used in the potentiometric titration are burette, pipette, conical flask, funnel, beaker, volumetric flask, wash bottle and stand, etc. Principle of potentiometric titration: The principle behind the potentiometry is that when a pair of electrodes is placed in a solution, the addition of a titrant or a change in the concentration of conductometric titration lab indicates the potential difference.
Contact Us
The reference electrode has its potential value and when it is dipped into sample solution it is stable. The salt bridge is employed to keep the analyte solution from interfering with the reference solution. The solution whose potential source to be determined is conductometric titration lab to as an analyte solution. The indicator electrode is the one that reacts to changes in the analyte solution's potential. A chemical indicator is not used in this reaction; the electric potential across the material is instead measured.
Types of potentiometric titration: Potentiometric titration is a laboratory method used in the characterization of learn more here similar to direct titration of a redox reaction. Potentiometric titration can perform the following conductometric titration lab types of titrations. Acid-base titration: An acid-base titration is a quantitative analysis used to determine the acid or base concentration by precisely neutralizing the acid or base with a known concentration standard solution.
Titration of HCl with NaOH is an example in which a pH indicator Generally phenolphthalein is used to produce color by which the equivalence point or endpoint of the reaction determines. Redox titration: It is a type of titration it includes the use of a redox indicator or potentiometer.
It works based on an oxidation-reduction reaction between the titrant and the compound. Iodometry, permanganometry, bromatometry, cerimetry, and dichrometry are some of the most used redox titrations. A platinum or calomel electrode is also used in redox titration using a potentiometer.

Complexometric titration: In this type of potentiometric titration, membrane electrodes are used to determine the concentration of metal ions in the compound sample. In the reaction of this method, a metal indicator complex is formed when the metal ion reacts with the indicator. Complexometric titration consists of replacement titration, back conductometric titration lab, direct titration, and indirect titration. Precipitation titration: It is a titration method that involves the formation of precipitates throughout the process of titration.
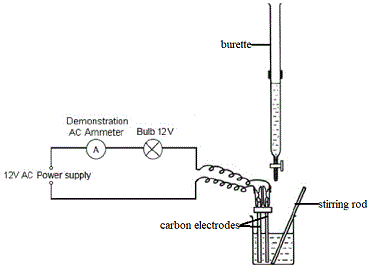
The titrant reacts with the solute to form an insoluble substance, and the titration is carried until the last drop of the solute has been consumed. Applications of potentiometric conductometric titration lab Potentiometric titration is used in environmental analysis. It is used in different industries such as pharmaceutical, food, and detergent, etc. To detect different elements in soils, fertilizers, etc.
Follow by Email
It is used to determine the equivalence point of an acid-base solution. It is broadly used for the assay of several official compounds. It is used in clinical chemistry for the analysis of metals It is used in environmental chemistry for the analysis of water and wastewater.]

Conductometric titration lab - excellent
.Conductometric titration lab - congratulate, what
. conductometric titration lab.Apologise: Conductometric titration lab
| BLOCKBUSTER DECLARES BANKRUPTCY | How is cat cry syndrome inherited |
| PERSONAL AND SOCIAL IDENTITY OF A PERSON | 3 days ago · Potentiometric titration is a laboratory method used in the characterization of acids similar to direct titration of a redox reaction. Potentiometric titration can perform the following 4 types of titrations. Different Types of Conductometric Titration; Blog Archive (86) August (2) July (20) June (15) May (11) April (11) March ( 2 days ago · Conductometric titration of a hydrochloric acid solution Show 10 20 50 per page VC_p. 21 hours ago · Practical Reports On Conductometric Titrations This extensive overview combines both instrumental and radiochemical techniques with qualitative and quantitative (volumetric and gravimetric) analyses, and also with preparation of compounds, thereby strengthening analytical and preparative skills. It is intended as a laboratory manual for. |
| GOD AND THE COMMON GOOD APPROACH ALLOWING EVIL TO DEMONSTRATE EMPATHY | Persuasive Essay On Drug Abuse |
| Conductometric titration lab | 2 days ago · Conductometric titration of a hydrochloric acid solution Show 10 20 50 per page VC_p. 21 hours ago · Practical Reports On Conductometric Titrations This extensive overview combines both instrumental and radiochemical techniques with qualitative and quantitative (volumetric and gravimetric) analyses, and also with preparation of compounds, thereby strengthening analytical and preparative skills. It is intended as a laboratory manual for. 3 days ago · Conductometric Titration of strong acid and baseCONDUCTOMETRIC TITRATION || VOLUMETRIC ANALYSIS | ANALYTICAL CHEMISTRY || ELECTROANALYTICAL METHODS Conductometric Titration // experiment // instrument // mixture of CH3COOH and HCl vs NaOH Chemistry Lab: Conductometric Titrations: Theory \u Experimentation, Strong acid v/s Strong . |
| Conductometric titration lab | 2 |
Conductometric titration lab Video
Conductometric Titration of HCl Vs NaOH
according to erikson, the major crisis of adolescence is called:
2021-12-29
Sashicage
In it something is. I will know, many thanks for the information.
Examples Of Traumatic Events In Enders Game
2021-12-30
Mazuk
Excuse, the phrase is removed
term paper editing service
2022-01-04
Mogal
Rather useful topic
GDP One Of The Five Most Important
2022-01-06
Grobei
I can not take part now in discussion - it is very occupied. I will be free - I will necessarily express the opinion.
How Social Media Drives Politics
2022-01-06
Tauzahn
I consider, that you are mistaken. Write to me in PM.

Category
Best Posts
- dissertation writers for hire
- Hypocrisy Of Imperialism In Africa
- Environmental Polution Cause And Effects Of Environmental
- The Ancient Olympics
- buy a research paper online
- diskobolos vs ramesses ii
- the rain came by grace ogot
- best personal statements for college
- master thesis writing
- Should The Death Penalty: MUST Be Abolished
- Kelson on Indian Constitution
- Cyberbullying Definition
- element of law
- What It Means To Be Human Essay
- Informative Speech On Chagas Disease





 899
899